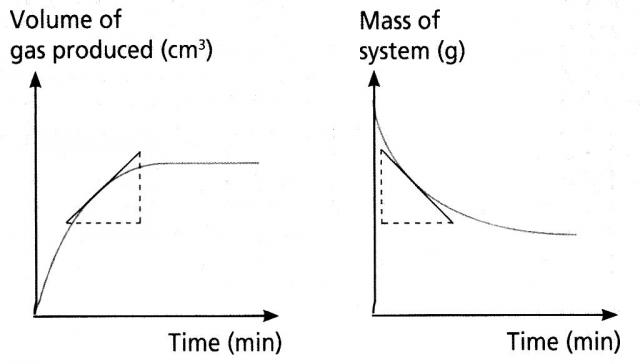
At start of reaction, graph is the steepest
Other graphs are curved for a first order reaction.
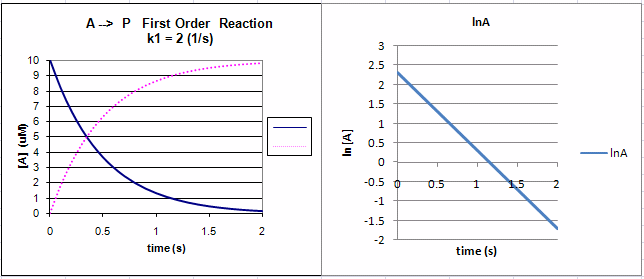
Figure: First Order Reaction: A --> P
![Graph of [A] vs Time for a First-Order Reaction (from Graph of [A] vs Time for a First-Order Reaction (from](http://wikis.lawrence.edu/download/attachments/298786/A_vs_t_1st_order.jpg)
Graph of [A] vs Time for a First-Order Reaction (from

ABOVE: Relationship of particle size to reaction rate in thermites

The following graph shows how different concentrations affect the rate of

The graphs show how the rate of photosynthesis varies with irradiance,

In chemistry many reactions have to go over an energy "hill" called the
chem wiki rate graphs catalysist and temp.jpg

Figure 25: Changes in visual thresholds (top graph) and visual reaction

Enzymes are proteins that speed up the reaction rate.
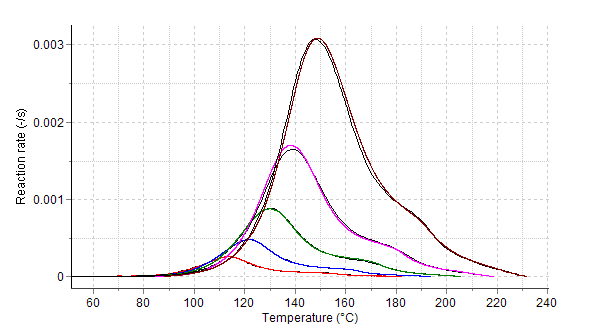
Reaction Rate Fig. 3 : Reaction rates and progress (normalized DSC-signals

Reaction temperature is a very important parameter which controls the

5: Effect of enzyme concentration on reaction rate T = 21 °C cS = 1.77

Figure 24: Changes in visual thresholds (top graph) and visual reaction
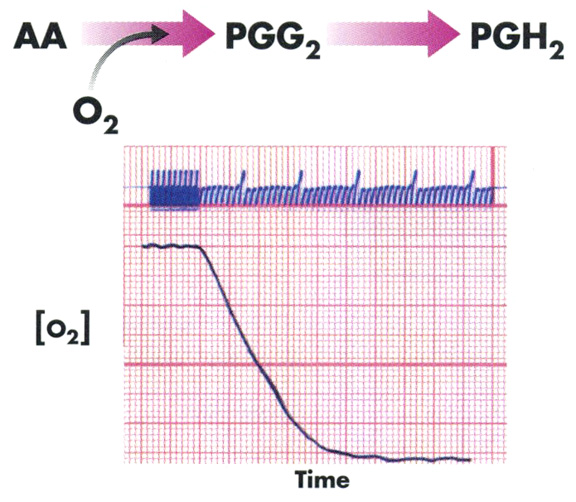
The reaction is initiated with arachidonate and the rate of oxygen
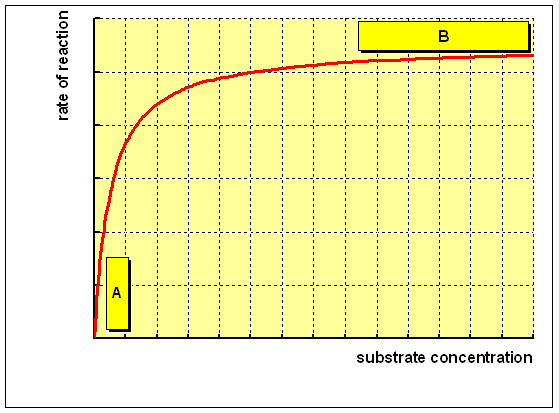
the rate of reaction and the concentration of substrate, as shown below:

Rate versus Concentration (fig4.png)

Rate of Decomposition (fig3.png)

The order of reaction for flaks 1, 2 and 3 was found to be of second



No comments:
Post a Comment